Systematic & scoping reviews
Systematic reviews
From Munn et al (2018): “Systematic reviews can be broadly defined as a type of research synthesis that are conducted by review groups with specialized skills, who set out to identify and retrieve international evidence that is relevant to a particular question or questions and to appraise and synthesize the results of this search to inform practice, policy and in some cases, further research. .. Systematic reviews follow a structured and pre-defined process that requires rigorous methods to ensure that the results are both reliable and meaningful to end users. .. A systematic review may be undertaken to confirm or refute whether or not current practice is based on relevant evidence, to establish the quality of that evidence, and to address any uncertainty or variation in practice that may be occurring. .. Conducting a systematic review may also identify gaps, deficiencies, and trends in the current evidence and can help underpin and inform future research in the area. .. Indications for systematic reviews are:
- Uncover the international evidence
- Confirm current practice/ address any variation/ identify new practices
- Identify and inform areas for future research
- Identify and investigate conflicting results
- Produce statements to guide decision-making”
Scoping reviews
From Munn et al (2018): “Scoping reviews are an ideal tool to determine the scope or coverage of a body of literature on a given topic and give clear indication of the volume of literature and studies available as well as an overview (broad or detailed) of its focus. Scoping reviews are useful for examining emerging evidence when it is still unclear what other, more specific questions can be posed and valuably addressed by a more precise systematic review. They can report on the types of evidence that address and inform practice in the field and the way the research has been conducted. The general purpose for conducting scoping reviews is to identify and map the available evidence. Purposes for conducting a scoping review:
- To identify the types of available evidence in a given field
- To clarify key concepts/ definitions in the literature
- To examine how research is conducted on a certain topic or field
- To identify key characteristics or factors related to a concept
- As a precursor to a systematic review
- To identify and analyse knowledge gaps”
Munn, Z., Peters, M. D. J., Stern, C., Tufanaru, C., McArthur, A., & Aromataris, E. (2018). Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Medical Research Methodology, 18(1), 143. https://doi.org/10.1186/s12874-018-0611-x
Reviews can be quantitative or qualitative
A quantitative review will include studies that have numerical data.
A qualitative review derives data from observation, interviews, or verbal interactions and focuses on the meanings and interpretations of the participants. It will include focus groups, interviews, observations and diaries. See the qualitative research section for more information.
PRISMA Statement
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses.
The PRISMA 2020 statement was published in 2021 and comprises a 27-item checklist addressing the introduction, methods, results and discussion sections of a systematic review report. It is intended to be accompanied by the PRISMA 2020 Explanation and Elaboration document.
The PRISMA extension for scoping reviews (PRISMA-ScR) was published in 2018. The checklist contains 20 essential reporting items and 2 optional items to include when completing a scoping review.
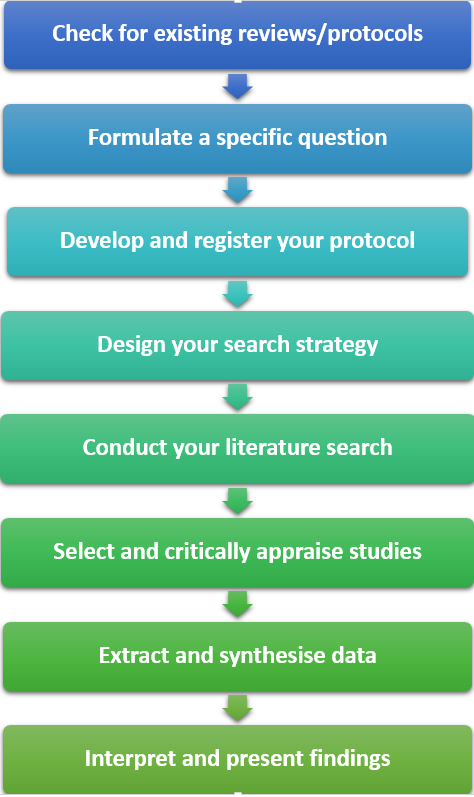
Steps in a systematic review
A systematic review involves the following steps:
- Check for existing reviews/protocols. If a systematic review answering your question has been conducted, or is being undertaken, you may need to amend or refine your question
- Formulate a specific research question that is clear and focused. Use the PICO tool (for quantitative reviews) or PICo (for qualitative reviews)
- Develop and register your protocol, including the rationale for the review, and eligibility criteria
- Design a robust search strategy that is explicit and reproducible
- Conduct a comprehensive search of the literature by searching the relevant databases and other sources
- Select and critically appraise the quality of included studies
- Extract relevant data from individual studies and use established methods to synthesise the data
- Interpret your results and prepare a comprehensive report on all aspects of your systematic review. Present your findings so that they can be translated into clinical practice.
Comparison of different types of reviews
This table outlines the differences between a systematic review and a literature review:
| Systematic Review | Literature Review | |
|---|---|---|
| Question | Focused on a single question | Not necessarily focused on a single question, but may describe an overview |
| Protocol | Includes a peer review protocol or plan | No protocol is included |
| Background | Provides summaries of the available literature on a topic | Provides summaries of the available literature on a topic |
| Objectives | Clear objectives are identified | Objectives may or may not be identified |
| Inclusion/exclusion criteria | Criteria is stated before review is conducted | Criteria is not specified |
| Search strategy | Comprehensive search conducted in a systematic way | Strategy not explicitly stated |
| Process of selecting articles | Process usually clear and explicit | Not described in a literature review |
| Process of evaluating articles | Comprehensive evaluation of study quality | Evaluation of study quality may or may not be included |
| Results and data synthesis | Clear summaries based on high quality evidence | Summary based on studies where the quality of the articles may not be specified. May also be influenced by the reviewer’s theories, needs and beliefs |
| Discussion | Written by an expert or group of experts with a detailed and well grounded knowledge of the issues | Written by an expert or group of experts with a well grounded knowledge of the issues |
Adapted from: University of Newcastle Australia Library
This table outlines the differences between a systematic review and a scoping review:
| Systematic Review | Scoping Review | |
|---|---|---|
| What is it? | Attempts to identify, appraise and synthesize all empirical evidence that meets pre-specified eligibility criteria to answer a given research question | A rapid gathering of literature in a given area, aiming to provide an overview of the type, extent and quantity of research available |
| Why choose this method? | To address a clearly focused review question by finding the best available, relevant studies and synthesizing the results | To capture the breadth of literature; identify gaps in a research area; occasionally used as a precursor to a systematic review |
| Question | Focused research question with narrow parameters | The research question is often broad |
| Eligibility criteria | Inclusion/exclusion usually defined at outset | Inclusion/exclusion can be developed post hoc |
| Appraisal | Rigorous critical appraisal and evaluation of study quality | Appraisal can be variable; typically not done, or may be done in a narrative form |
| Synthesis | Clear summaries of studies based on high quality evidence. May include a meta-analysis | The summary is usually descriptive |
| Inferences | Evidence based | Evidence based |
Adapted from: University of South Australia
References:
Munn, Z., Peters, M. D. J., Stern, C., Tufanaru, C., McArthur, A., & Aromataris, E. (2018). Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Medical Research Methodology, 18(1), 143. https://doi.org/10.1186/s12874-018-0611-x
Pollock, D., Davies, E. L., Peters, M. D. J., et al. (2021). Undertaking a scoping review: A practical guide for nursing and midwifery students, clinicians, researchers, and academics. J Adv Nurs, 77, 2102-2113. https://onlinelibrary.wiley.com/doi/10.1111/jan.14743
“Rapid reviews have emerged as a streamlined approach to synthesizing evidence-typically for informing emergent decisions faced by decision makers in health care setting”.
| Systematic Review | Rapid Review | |
|---|---|---|
| Question | Often a focused clinical question (focused PICOS) | Narrow question (may use PICOS) |
| Sources and searches | Comprehensive sources searched and explicit strategies | Sources may be limited but sources and strategies made explicit |
| Selection | Criterion-based | Criterion-based; uniformly applied |
| Appraisal | Rigorous; critical appraisal | Rigorous, critical appraisal (SRs only) |
| Synthesis | Qualitative summary with/without meta-analysis | Descriptive summary/categorisation of data |
| Inferences | Evidence-based | Limited/cautious interpretation of findings |
Source: Khangura, S., Konyu, K., Cushman, R., Grimshaw, J. & Moher, D. (2012). Evidence summaries: the evolution of a rapid review approach. Systematic Review, 1-10. https://doi.org/10.1186/2046-4053-1-10
Examples of different types of reviews:
Literature review:
A Literature review of mentorship programs in academic nursing
https://doi.org/10.1016/j.profnurs.2017.02.007
Narrative review:
A silent burden—prolapse, incontinence, and infertility in Australian Aboriginal and Torres Strait Islander women: A systematic search and narrative review
https://doi.org/10.1002/ijgo.13920
Rapid review:
Blended foods for tube-fed children: a safe and realistic option? A rapid review of the evidence
https://doi.org/10.1136/archdischild-2016-311030
Scoping review:
How do patients experience caring? Scoping review
https://doi.org/10.1016/j.pec.2017.03.029
Systematic review:
Barriers and facilitators to health screening in men: A systematic review
https://doi.org/10.1016/j.socscimed.2016.07.023
A typology of reviews: an analysis of 14 review types and associated methodologies (2009) https://onlinelibrary.wiley.com/doi/10.1111/j.1471-1842.2009.00848.x